国产不卡视频一区二区三区,欧美日韩在线视频,成全免费高清大全,国产精品久久毛片

13批次藥品不合格,涉江西齊仁堂等企業
1月24日,國家藥監局網站發布關于13批次藥品不符合規定的通告。通告提到,經安徽省食品藥品檢驗研究院等7家藥品檢驗機構檢驗,共10家企業生產的13批次藥品不符合規定。
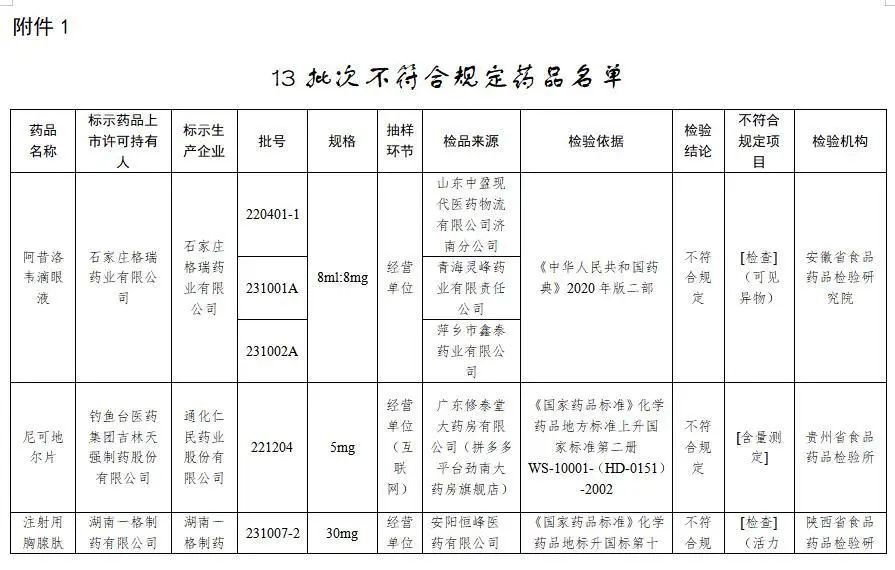
一、經安徽省食品藥品檢驗研究院檢驗,標示為石家莊格瑞藥業有限公司生產的3批次阿昔洛韋滴眼液不符合規定,不符合規定項目為可見異物。
二、經貴州省食品藥品檢驗所檢驗,標示為通化仁民藥業股份有限公司生產的1批次尼可地爾片不符合規定,不符合規定項目為含量測定。
三、經陜西省食品藥品檢驗研究院檢驗,標示為湖南一格制藥有限公司生產的2批次注射用胸腺肽不符合規定,不符合規定項目為活力測定。
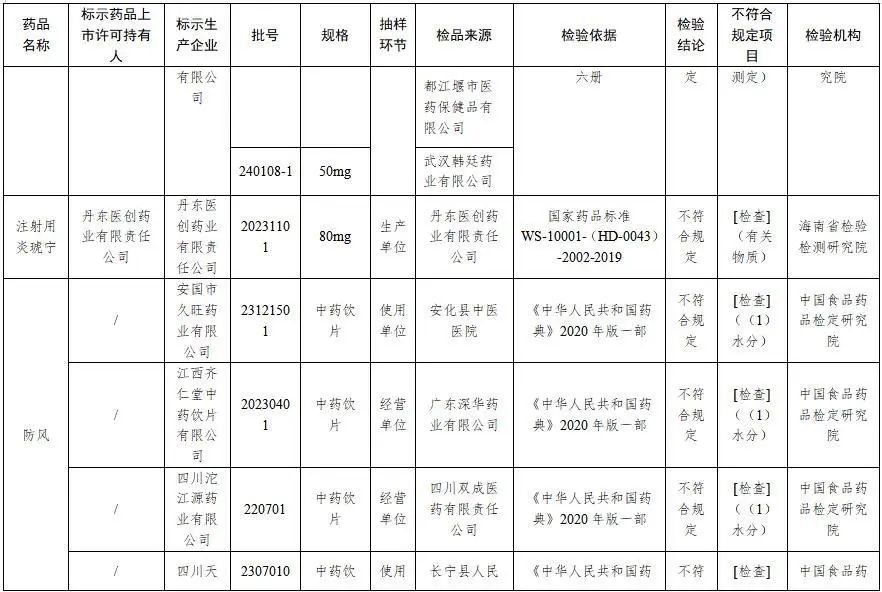
四、經海南省檢驗檢測研究院檢驗,標示為丹東醫創藥業有限責任公司生產的1批次注射用炎琥寧不符合規定,不符合規定項目為有關物質。
五、經中國食品藥品檢定研究院檢驗,標示為安國市久旺藥業有限公司、江西齊仁堂中藥飲片有限公司、四川沱江源藥業有限公司、四川天植中藥股份有限公司生產的4批次防風不符合規定,不符合規定項目為水分。
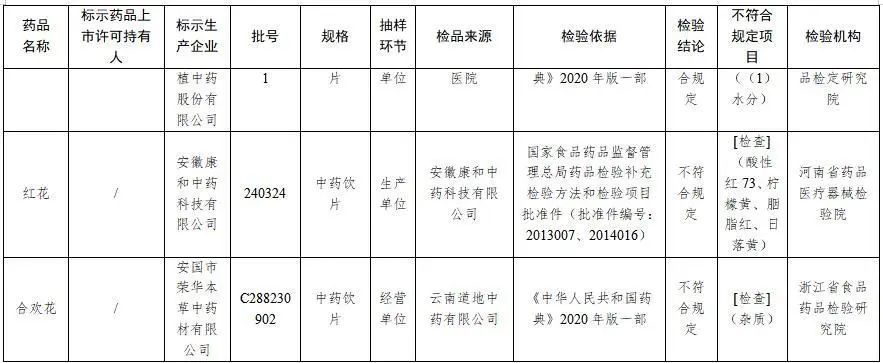
六、經河南省藥品醫療器械檢驗院檢驗,標示為安徽康和中藥科技有限公司生產的1批次紅花不符合規定,不符合規定項目為酸性紅73、檸檬黃、胭脂紅、日落黃。
七、經浙江省食品藥品檢驗研究院檢驗,標示為安國市榮華本草中藥材有限公司生產的1批次合歡花不符合規定,不符合規定項目為雜質。
13批次不符合規定藥品名單
國家藥監局表示,對上述不符合規定藥品,藥品監管部門已要求相關企業和單位采取暫停銷售使用、召回等風險控制措施,對不符合規定原因開展調查并切實進行整改。國家藥監局要求相關省級藥品監管部門依據《中華人民共和國藥品管理法》,組織對上述企業和單位存在的涉嫌違法行為立案調查,并按規定公開查處結果。
來源:中新經緯




